
Z Is An Anhydrous Compound Of A Group 2 Element When It Is Heated Z Undergoes Thermal Decomposition To Produce Two Different Gases Z Has Relatively Low Thermal Stablity Compared To Other
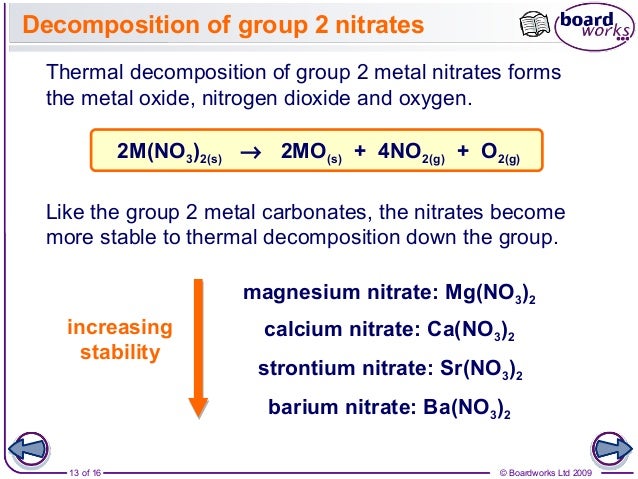
Trends In Group 2 Part 3 Chemical Properties
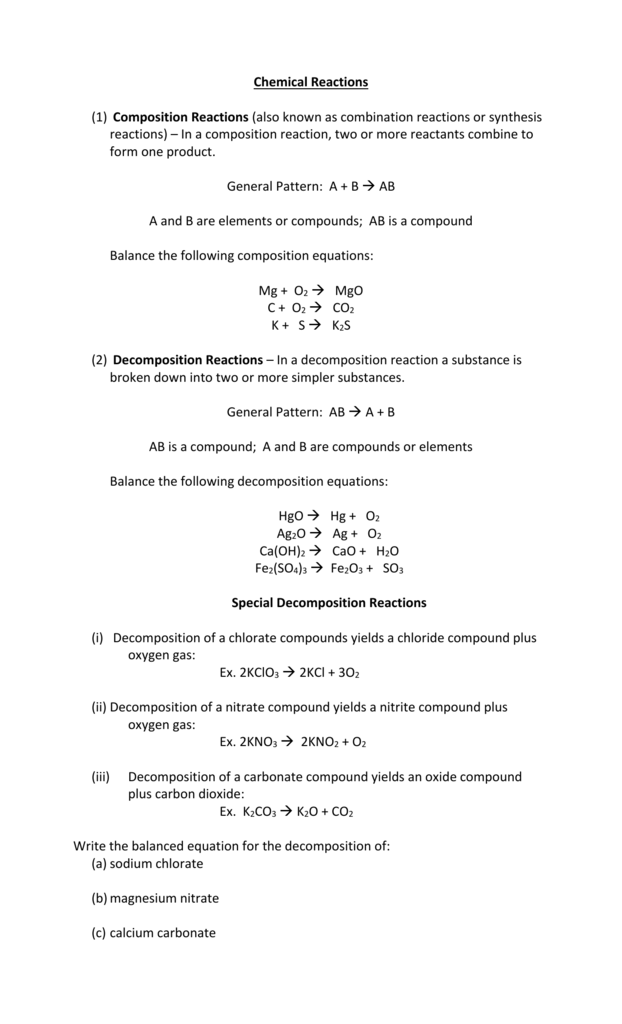
Chemical Equations

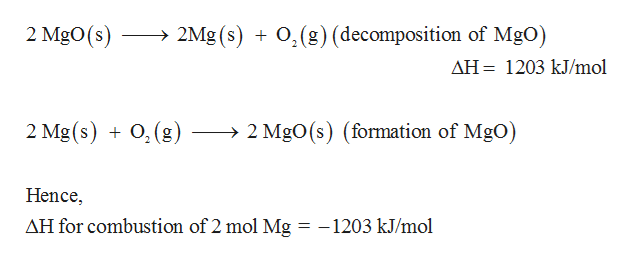
Answered The Endothermic Decomposition Of Bartleby

Edexcel Jun 2016 Paper 2 Q15 With Explained Solutions
Solved This Questions Is Regarding Numbers 5 And 7 In The Chegg Com
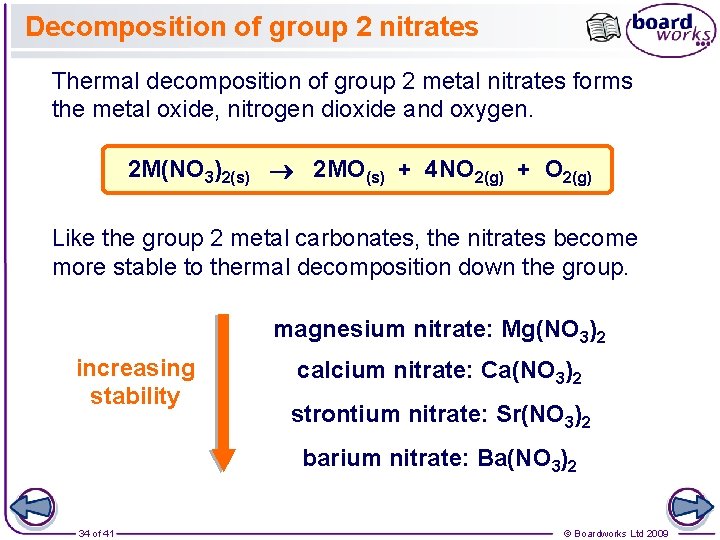
1 Of 41 Boardworks Ltd 2009 2 Of
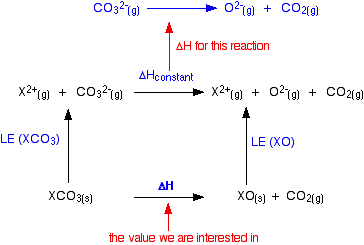
Thermal Decomposition Of The Group 2 Carbonates And Nitrates
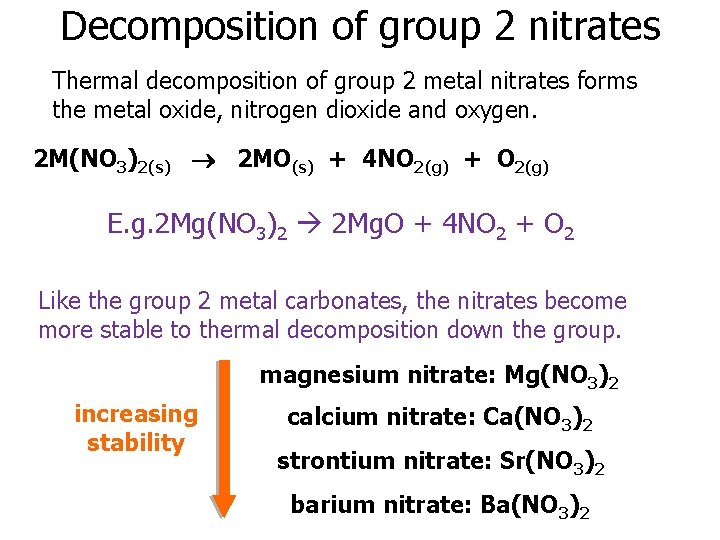
Groups 1 And 2 Compounds 09 November 2020
Thermal Stability Of Group 2 Nitrates
Buy Magnesium Nitrate Hexahydrate 215530290 In India Biomall
Tags:
Archive

